Precision Calibration and Cutting Machine for Pharmaceutical Micro-Implants
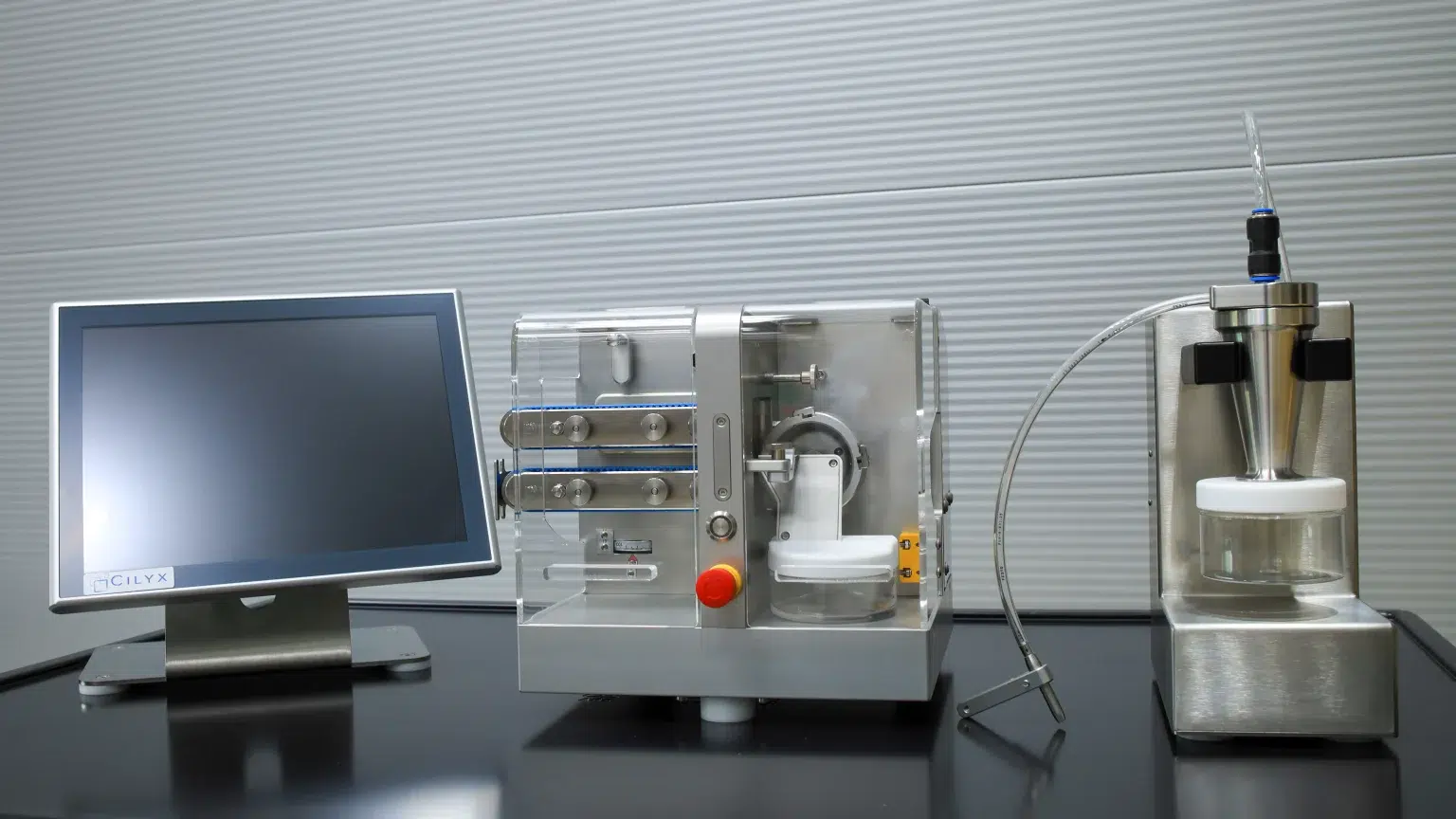
New drug delivery systems, such as ocular micro-implants or subcutaneous micro-implants, require precise production with tight tolerances for the diameter and length of polymer strands. The MicroCut post-extrusion system is the next generation of instruments for the precise calibration of polymer strands and cutting them into well-defined micro-implants. Suitable for a variety of polymer formulations and application areas, the MicroCut post-extrusion system is a highly modular instrument that can be adjusted to meet a wide range of product specifications.
High Precision
The compact MicroCut post-extrusion system is placed right next to the extruder die, so the polymer strand can be directly guided through a 1D laser diameter gauge and onto the conveyor without significant cooling. Based on the laser measurement, the conveyor speed is adjusted and pulls the strand, allowing the diameter to be adjusted within defined tolerances. Depending on the extruder die, the MicroCut system can handle a nominal strand diameter of 0.2 mm to 1.4 mm. The nominal length of a micro-implant can be set to a minimum of 1 mm.
Maximum Flexibility
Before a strand enters the cutting section of the instrument, the length of the conveyor ensures sufficient cooling of the strand, allowing for cutting with maximum precision. To meet a wide range of polymer formulations and throughput needs, the instrument is available with conveyor lengths of 350 mm, 700 mm, or even 1200 mm. Cooling can be accelerated with compressed air.
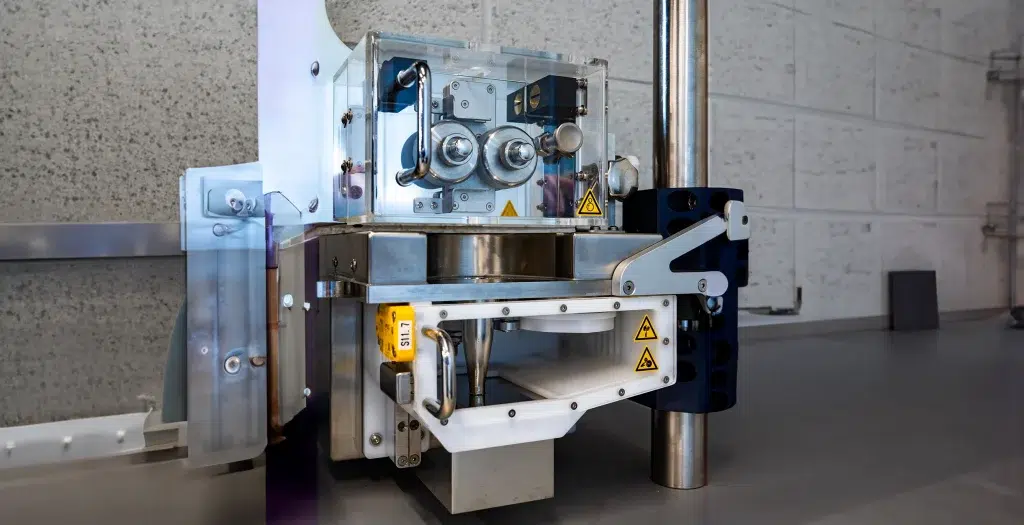
The cutting of a micro-implant is a crucial step, and smooth cutting surfaces depend greatly on the polymer formulation and the cutting method applied. Therefore, the MicroCut post-extrusion system can be equipped with two different cutting mechanisms: a rotary blade and a linear blade. Both cutters can be parameterized to ensure the best possible cutting for all micro-implant materials. Adjustment possibilities for different cutting cone geometries allow for cutting solutions for diverse product qualities. As a production device, the MicroCut system is suitable for up to 600 cuts/min.
Pharmaceutical Application
The computer control of the MicroCut system is housed in a rugged stainless steel enclosure that complies with GMP guidelines. It can be placed on or next to the instrument to accommodate the best possible production line layout. The user interface is easy to operate, allows full access to all machine parameters, and monitors the analytical performance with a live diagram of the measured strand diameter. The software features different user levels with customizable access rights, secure data export, and audit trail support for 21 CFR Part 11 compliance. The MicroCut post-extrusion system comes with complete IQ/OQ documentation.
Thanks to its great flexibility and interchangeable parts, the MicroCut system can be configured for use in research and development as well as commercial manufacturing.